| Serial NO. | GA-E0937HM |
| Species | Human |
| Specifications | 96T/48T |
| Brand | GenAsia |
| Origin | Shanghai China |
| Price | US$388/US$200 |
Detailed information
[Specificity]
This assay has high sensitivity and excellent specificity for detection of Human CAD. No significant cross-reactivity or interference between Human CAD and analogues was observed.
[Precision]
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Human CAD were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Human CAD were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
[Detection Wavelength] 450 nm
[Assay Time] 1-2h
[Sample Volume]: 50-100ul
[Intended Use] For research use only. Not for diagnostic use.
[Specimen Requrements]
a) Samples containing NaN3 shall not be tested as they inhibit the activity of Horse Radish Peroxidase (HRP).
b) Upon sample collection, extraction should be carried out as soon as possible in accordance with related documents. After extraction, the experiment should be conducted immediately as well. Otherwise, the sample should be preserved at -20℃. Repeated freeze-thaw cycles should be avoided.
c) Serum: Allow the serum to clot for 10-20 minutes at room temperature, then place in centrifuge (at 2000-3000 RPM) for approximately 20 minutes. Collect the supernatants carefully. If sediments have occurred during storage, centrifugation should be repeated.
d) Blood Plasma: In accordance with sample collection requirements, EDTA or sodium citrate should be used for anticoagulation. Add EDTA or sodium citrate and mix them for 10-20 minutes, then place in centrifuge(at 2000-3000 RPM) for approximately 20 minutes. Collect supernatants carefully. If sediments have occurred during storage, centrifugation should berepeated.
e) Urine: Collect with sterile tube. Place in centrifuge (at 2000-3000 RPM) for approximately 20 minutes. Collect the supernatants carefully. If sediments have occurred during storage, centrifugation should be repeated.
When collecting pleuroperitoneal fluid and cerebrospinal fluid, please follow the procedures mentioned above.
f) Cell Culture Supernatant: Collect with sterile tubes when examining secrete components. Place in centrifuge (at 2000-3000 RPM) for approximately 20 minutes. Collect the supernatants carefully. When examining the components within the cell, use PBS (PH 7.2-7.4) to dilute cell suspension to cell concentration of approximately 1 million/ml. Degrade cells through repeated freeze-thaw cycles to release interior components. Place in centrifuge (at 2000-3000 RPM) for approximately 20 minutes. Collect the supernatants carefully. If sediments have occurred during storage, centrifugation should be repeated.
g) Tissue Sample: Incise sample and weigh. Add a given amount of PBS (PH 7.4). Immediately freeze with liquid nitrogen for later use. Thaw sample and hold at 2-8℃. Add a given amount of PBS (PH 7.4), then homogenize the sample thoroughly by hand or with homogenizer. Place in centrifuge (at 2000-3000 RPM) for approximately 20 minutes. Collect the supernatants carefully. Aliquot and keep one for examination and freeze the others for later use.
[Notes]
a) Hold kit at room temperature for at least 30 minutes once removed from 2-8℃ environment. Coated ELISA plates should be stored in sealed bag if not used after opening.
b) When adding samples, sample injector must be used each time and should also be frequently checked for precision to avoid individual error.
c) The operating instructions must be strictly followed and the reading of ELISA reader must be set as the standard for determining the experiment result.
d) Pipette tips and seal plate membrane in hand should not be used more than once in order to avoid cross contamination.
e) All samples, washing concentration, and all waste should be disposed as infective agents.
f) Other reagents not needed shall be packed or covered. Reagents of different batches shall not be mixed and should be used before their respective validity dates.
g) Substrate B is sensitive to light and therefore should not be over-exposed to light.
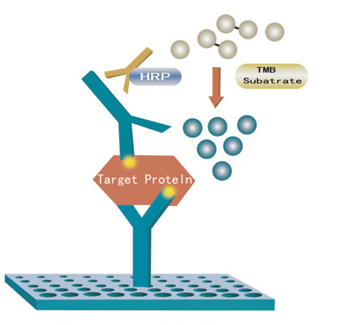
[Assay Procedure]
a) Dilution of standard solutions: (This kit provides one standard original concentration. Users may independently dilute in small tubes following the manual.) b) The number of stripes needed is determined by that of samples to be tested added by the standards. It is recommended that each standard solution and each blank well be arranged with multiple wells as much as possible.
c) Sample injection: 1) Blank well: no sample, anti Human CAD antibody labeled with biotin or Streptavidin-HRP is added to comparison blank well; add chromogen solution A & B and stop solution, each other step operation is the same. 2) Standard solution well: Add 50μl standard and streptomycin-HRP 50μl (biotin antibodies have united in advance in the standard so no biotin antibodies are added). 3) Sample well to be tested: Add 40μl sample and then 10μl Human CAD antibodies, 50μl Streptavidin-HRP. Then cover it with seal plate membrane. Shake gently to mix. Incubate at 37℃ for 60 minutes.
d) Preparation of washing solution: Dilute the washing concentration (30X) with distilled water for later use.
e) Washing: carefully remove the seal plate membrane, drain liquid and shake off the remainder. Fill each well with washing solution, let stand for 30 seconds, then drain. Repeat this procedure five times then blot the plate.
f) Color development: First add 50μl chromogen solution A to each well, and then add 50μl chromogen solution B to each well. Shake gently to mix. Incubate for 10 minutes at 37℃ away from light for color development.
g) Stop: Add 50μl Stop Solution to each well to stop the reaction (color changes from blue to yellow immediately at that moment).
h) Assay: Take blank well as zero, measure the absorbance (OD) of each well one by one under 450nm wavelength, which should be conducted within10 minutes after having added stop solution.
i) According to standards concentrations and corresponding OD values, calculate the linear regression equation of the standard curve. Then according to the OD value of samples, calculate the concentration of the corresponding sample. Statistical software could also be employed.
[Calculation]
Make concentration of standards the abscissa and OD value the ordinate. Draw the standard curve on the graph paper. According to the OD value of the sample, locate its corresponding concentration (which is the concentration of the sample); or calculate the linear regression equation of the standard curve according to the standard concentration and the OD value. Then substitute with the OD value of the sample to calculate its concentration.
This assay has high sensitivity and excellent specificity for detection of Human CAD. No significant cross-reactivity or interference between Human CAD and analogues was observed.
[Precision]
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Human CAD were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Human CAD were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
[Detection Wavelength] 450 nm
[Assay Time] 1-2h
[Sample Volume]: 50-100ul
[Intended Use] For research use only. Not for diagnostic use.
[Specimen Requrements]
a) Samples containing NaN3 shall not be tested as they inhibit the activity of Horse Radish Peroxidase (HRP).
b) Upon sample collection, extraction should be carried out as soon as possible in accordance with related documents. After extraction, the experiment should be conducted immediately as well. Otherwise, the sample should be preserved at -20℃. Repeated freeze-thaw cycles should be avoided.
c) Serum: Allow the serum to clot for 10-20 minutes at room temperature, then place in centrifuge (at 2000-3000 RPM) for approximately 20 minutes. Collect the supernatants carefully. If sediments have occurred during storage, centrifugation should be repeated.
d) Blood Plasma: In accordance with sample collection requirements, EDTA or sodium citrate should be used for anticoagulation. Add EDTA or sodium citrate and mix them for 10-20 minutes, then place in centrifuge(at 2000-3000 RPM) for approximately 20 minutes. Collect supernatants carefully. If sediments have occurred during storage, centrifugation should berepeated.
e) Urine: Collect with sterile tube. Place in centrifuge (at 2000-3000 RPM) for approximately 20 minutes. Collect the supernatants carefully. If sediments have occurred during storage, centrifugation should be repeated.
When collecting pleuroperitoneal fluid and cerebrospinal fluid, please follow the procedures mentioned above.
f) Cell Culture Supernatant: Collect with sterile tubes when examining secrete components. Place in centrifuge (at 2000-3000 RPM) for approximately 20 minutes. Collect the supernatants carefully. When examining the components within the cell, use PBS (PH 7.2-7.4) to dilute cell suspension to cell concentration of approximately 1 million/ml. Degrade cells through repeated freeze-thaw cycles to release interior components. Place in centrifuge (at 2000-3000 RPM) for approximately 20 minutes. Collect the supernatants carefully. If sediments have occurred during storage, centrifugation should be repeated.
g) Tissue Sample: Incise sample and weigh. Add a given amount of PBS (PH 7.4). Immediately freeze with liquid nitrogen for later use. Thaw sample and hold at 2-8℃. Add a given amount of PBS (PH 7.4), then homogenize the sample thoroughly by hand or with homogenizer. Place in centrifuge (at 2000-3000 RPM) for approximately 20 minutes. Collect the supernatants carefully. Aliquot and keep one for examination and freeze the others for later use.
[Notes]
a) Hold kit at room temperature for at least 30 minutes once removed from 2-8℃ environment. Coated ELISA plates should be stored in sealed bag if not used after opening.
b) When adding samples, sample injector must be used each time and should also be frequently checked for precision to avoid individual error.
c) The operating instructions must be strictly followed and the reading of ELISA reader must be set as the standard for determining the experiment result.
d) Pipette tips and seal plate membrane in hand should not be used more than once in order to avoid cross contamination.
e) All samples, washing concentration, and all waste should be disposed as infective agents.
f) Other reagents not needed shall be packed or covered. Reagents of different batches shall not be mixed and should be used before their respective validity dates.
g) Substrate B is sensitive to light and therefore should not be over-exposed to light.
[Assay Procedure]
a) Dilution of standard solutions: (This kit provides one standard original concentration. Users may independently dilute in small tubes following the manual.) b) The number of stripes needed is determined by that of samples to be tested added by the standards. It is recommended that each standard solution and each blank well be arranged with multiple wells as much as possible.
c) Sample injection: 1) Blank well: no sample, anti Human CAD antibody labeled with biotin or Streptavidin-HRP is added to comparison blank well; add chromogen solution A & B and stop solution, each other step operation is the same. 2) Standard solution well: Add 50μl standard and streptomycin-HRP 50μl (biotin antibodies have united in advance in the standard so no biotin antibodies are added). 3) Sample well to be tested: Add 40μl sample and then 10μl Human CAD antibodies, 50μl Streptavidin-HRP. Then cover it with seal plate membrane. Shake gently to mix. Incubate at 37℃ for 60 minutes.
d) Preparation of washing solution: Dilute the washing concentration (30X) with distilled water for later use.
e) Washing: carefully remove the seal plate membrane, drain liquid and shake off the remainder. Fill each well with washing solution, let stand for 30 seconds, then drain. Repeat this procedure five times then blot the plate.
f) Color development: First add 50μl chromogen solution A to each well, and then add 50μl chromogen solution B to each well. Shake gently to mix. Incubate for 10 minutes at 37℃ away from light for color development.
g) Stop: Add 50μl Stop Solution to each well to stop the reaction (color changes from blue to yellow immediately at that moment).
h) Assay: Take blank well as zero, measure the absorbance (OD) of each well one by one under 450nm wavelength, which should be conducted within10 minutes after having added stop solution.
i) According to standards concentrations and corresponding OD values, calculate the linear regression equation of the standard curve. Then according to the OD value of samples, calculate the concentration of the corresponding sample. Statistical software could also be employed.
[Calculation]
Make concentration of standards the abscissa and OD value the ordinate. Draw the standard curve on the graph paper. According to the OD value of the sample, locate its corresponding concentration (which is the concentration of the sample); or calculate the linear regression equation of the standard curve according to the standard concentration and the OD value. Then substitute with the OD value of the sample to calculate its concentration.








